where the proportionality constant CB is NkT.
Jacques Charles discovered in 1780 that close to room temperature and at about one atmospheric pressure, the volume and temperature of gases are directly proportional to each other. Known today as Charles’s Law, which is written as
where the proportionality constant CC is Nk/P.
The Gay-Lussac’s Law states that close to room temperature and at about one atmospheric pressure, the pressure and temperature of gases are directly proportional to each other. It was discovered by Joseph Louis Gay-Lussac in 1809 and named after him. Gay-Lussac’s Law is written as
where the proportionality constant CG = Nk/V.
These laws can be explained if we assume that the gas is ideal. The ideal gas model is used to describe the behavior of dilute gases at low pressures and high temperatures. Many gases e.g., nitrogen, oxygen and hydrogen, can be considered to be ideal gases at room temperature and pressures close to 1 atm. Using this assumption, combining the three gas laws discuss above, which is discovered experimentally gives as the equation of the state of an ideal gas.
where P is the pressure, V is the volume, N is the number of particles and T is the temperature of the gas measured in the absolute or Kelvin scale. The constant k in the ideal gas equation is called the Boltzmann constant and has the value k = 1.3806488×10−23 m^2·kg/s^2·K^1.
In this experiment, we wish to verify the Boyle's Law and Charles's Law only and use the ideal gas equation to compute for the number of particles in the gas.
Boyle's Law
The height of the gas in the piston where recorded. The
volume of the cylinder Vcyl for each height was computed. The reciprocal P of
each pressure reading was computed. Plotting Vcyl vs P^-1 was done.
 |
| Table B1 |
Table 1 above shows us that as we increase the mass of the object that we place, the height decreases and the pressure increases as well.
 |
| Figure 1 |
The information that we got from the linear fit equation are
the following:
We obtain the volume of the chamber which is .0004 m^3, and also we can get the number of particle using the slope of the linear fit which is 1.0543E+22.
Charles's Law
The first step in this part of the experiment was to remove
the beaker (will serve as the hot bath) from the top of the stove. The
air chamber was placed in the hot bath. The pressure P was monitored throughout
the experiment. The temperature T of the hot bath was measured using a digital thermometer
and the initial height h of the piston was recorded. Chunks of ice were slowly
added on the hot bath while taking h and T measurements at equal time
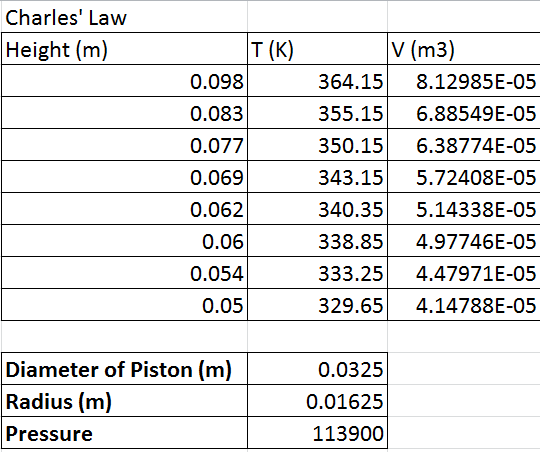
intervals. A graph of Vcham VS Temperature using the obtained data below. Also, the data from table 2 tells us that as the temperature
decreased, the height decreased which means that the volume decreased.
 |
| Table 2A |
 |
| Figure 2 |
 |
| Table 2B |
The number of particles N, which is 8.2487E+21 and the volume of
the air chamber can Vcham is .0003 m^3 were calculated from the slope and y-intercept,
respectively.
Conclusion: The data is precise because we have R^2 = .99 on both experiment. The data on the two experiment has only small discrepancy, since the theoretical number of particles is not given, the data obtained cannot be verified if its accurate. Over all, the experiment is well done with a small discrepancy.
Reference:
1. “Experiment 9 Gas Laws,” Laboratory Manual for Physics (Physics 73.1), (2013)





“When I was introduced to Mr. Lee Ben ( A Loan Officer), I was entering the market as a first time home buyer.My needs were a bit different and I had loads of questions, before he sent me my pre-approval letter, he called to speak with me about what it meant and what could change. He made himself available to me at pretty much any hour via email and texts, he was very responsive and knowledgeable. He’s also very straightforward, I explained to him what my expectations were in terms of closing time and other particulars. He said he would meet those expectations but he surpassed them. I closed so quickly my realtor and the seller of course were excited about that. But as a buyer I appreciated being walked through the process in a succinct yet thorough fashion. From pre-approval to closing- the journey was so seamless and I consider myself lucky because I’ve heard horror stories about the internet . I recommend A loan officer ben lee contact email: 247officedept@gmail.com & Whatsapp Number :+1-989-394-3740 to anyone looking for a loan in any market. Everything was handled electronically expediently and securely.
TumugonBurahin